Commercial coating formulations are rarely monopigmented; they are usually blends of two or more different pigments. Of course, all the pigments in these systems should also be wetted well and extensively deflocculated. However, this can cause another issue: ideally, all pigments are evenly distributed throughout the entire coating film; however, if this blend is disturbed, the pigments separate from each other, and this can cause color changes in the coating. This defect is known as “floating”.
One of the causes for the pigments to become separated from each other is flow effects in the dry coating film. Solvent must be transported from the lower coating layer to the surface. Evaporation causes the density of the remaining materials to increase and the solvent then sinks again.
In addition, evaporation causes cooling effects and the surface tension changes. All of this leads to the development of eddy currents which arrange themselves in the form of more or less equal hexagonal cells (so-called Bénard cells). In the center of the cells, the coating material rises and then distributes itself across the surface and then flows back down along the cell borders. These cell flows have been known for a long time – not just in coatings – and occur in every liquid coating film (even non-pigmented ones). In a pigmented system, the pigments also participate in these eddy currents and provided the mobility of the different pigments is similar, they are also transported into the eddy currents in a very similar manner and cannot be separated. If, however, the pigment mobility is considerably different, then the transport behavior is also different and can lead to separation.
Differences in the pigment mobility are often the main reason for pigments to no longer be homogeneously distributed. When solvents evaporate from a dry coating film, eddy currents (Bénard cells) form. Such movements cause small differences in temperature, density, and surface tension. The pigments participate in such movements, and the different pigment mobilities can cause the pigment to separate. This differential mobility can be equilibrated through the usage of controlled flocculating additives.
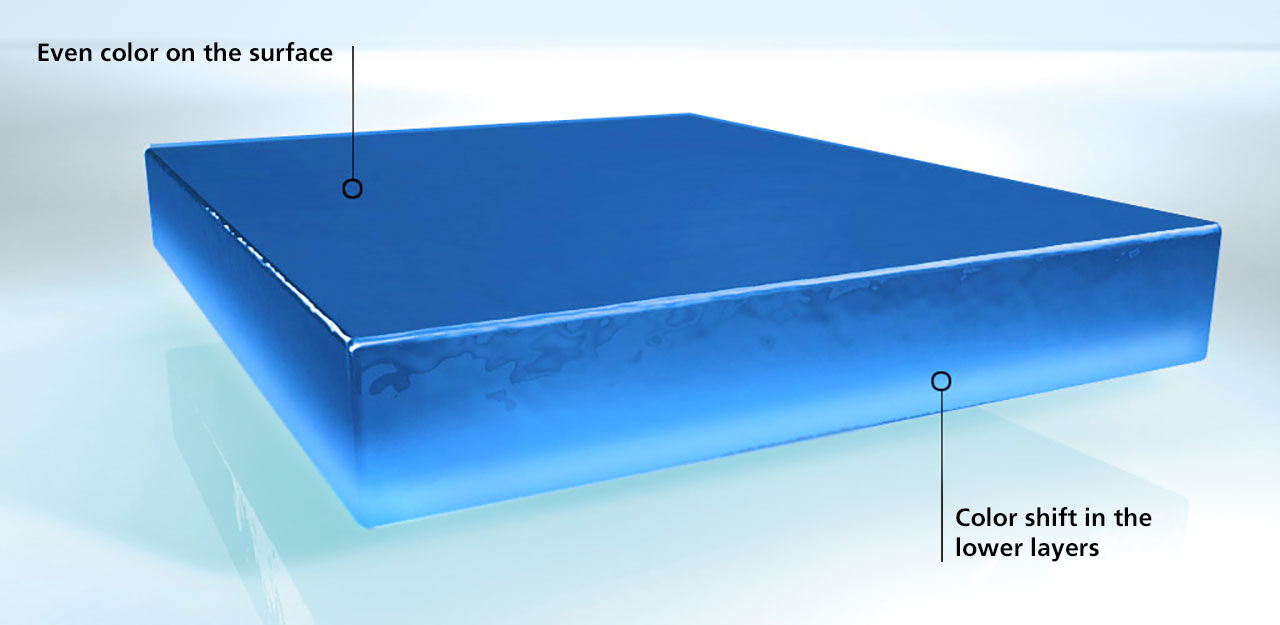
A pigment type has then concentrated itself on the surface and, in this case, the coating film has a uniform color on the surface (which, however, is not the original color of the pigment blend). The defect is only visible when the rub-up test is carried out. This test involves rubbing a small area of the still wet coating film with a finger, i.e. a homogenous blend of the different pigments is created in this area. The instability of the pigment blend is shown by the fact that a color difference is evident between the rubbed area and the surrounding coating material. This color difference can also be measured (as ∆E) and used as a quality criterion.
To avoid floating defects, we have to influence the mobility of the pigments. We have to make the mobilities of the various pigment types as similar as possible. One option is to use controlled flocculation. Controlled flocculating additives incorporate the different pigments together in the flocculate and therefore forcibly adjust their mobility. The targeted co-flocculation of the different pigment types therefore combats separation.
Of course, on account of the possible gloss reduction and more unfavorable pigment utilization, flocculation – even a controlled one – is not particularly desired in many topcoat systems, especially high-quality ones.
Polymeric wetting and dispersing additives can provide a solution here. They have proven performance in equilibrating pigment mobility, while simultaneously providing complete deflocculation of all pigments. Through the interaction of the adsorbed polymeric additives with the surrounding binder solution, the deflocculated pigment particles are tightly incorporated into the binder system and their mobility reduced.
This explains how floating can be eliminated even in the presence of smaller, deflocculated organic pigment particles and larger, inorganic pigments, as all the pigments have similar mobility.
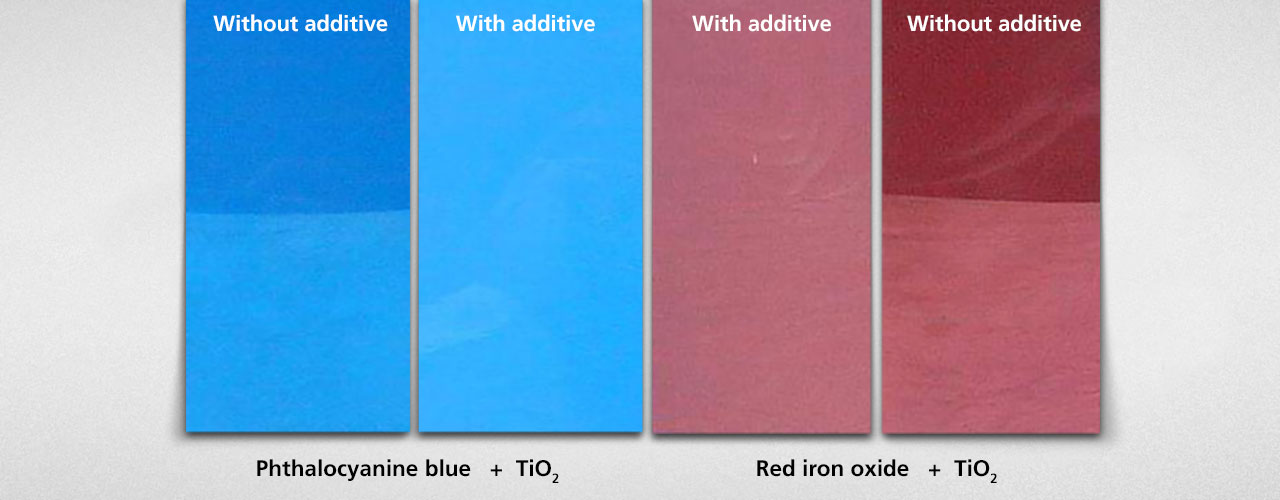
In non-aqueous systems, electrical charges on the pigment surface are usually so weak that they play no major part in stabilizing the pigments against flocculation. Unlike charges of the pigments can, however, have negative effects on the stability of pigment blends and therefore the floating behavior. The charge carried by a pigment depends not only upon the pigment itself, but also upon the binder solution: a pigment that is ground in different binders can display different charges. Different pigments that are ground in the same binder can also display different charges. Obviously, strong flocculation can occur when different pigment charges exist within the same coating.
It is important to note in practice that the pigment charge can also be influenced by the dispersing additive that is used: some polymeric additives are capable of equilibrating differently charged pigment particles in their electrical charge. The additive provides an additional stabilizing effect: the polymeric additive equilibrates not only the mobility of the various pigments (in addition to providing steric stabilization), but also ensures that all the pigment particles carry the same charge, thereby avoiding any instability due to charge differences.
On this website we use cookies and similar functions to process end device information and personal data. The processing is used for purposes such as to integrate content, external services and elements from third parties, statistical analysis/measurement, personalized advertising and the integration of social media. Depending on the function, data is passed on to up to 9 third parties and processed by them. This consent is voluntary, not required for the use of our website and can be revoked at any time using the icon on the bottom left.