
Instead of using electrical charge, a repulsion potential can also be developed between dispersed particles by means of the adsorbed polymer coatings on the surface. Each particle is surrounded by an envelope of solvated polymer molecules and, when approaching, two particles overlap and penetrate these polymer envelopes.
This increases the polymer concentration in the overlapping area and the osmotic pressure causes the solvent to be transported to this area so that the particles are repelled away from each other again. In addition, in the overlapping area the polymer molecules are restricted in their conformation, which signifies a reduction in the entropy and therefore represents a repulsion potential. Depending on the system, an enthalpic as well as an entropic contribution can be made towards stabilization.
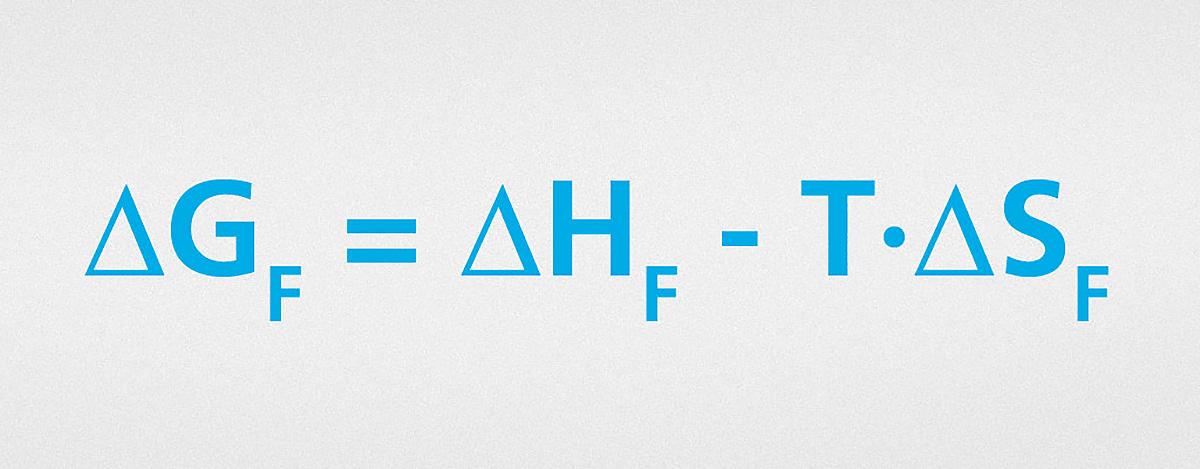
To prevent flocculation, the free energy, ΔGF, of this process must be positive. If both ΔHF and ΔSF are negative, yet the entropy term is greater than the enthalpy term, this results in a positive value for ΔGF and we have entropic stabilization. If ΔHF is positive, the system is truly stable and in this case we also have an enthalpic contribution towards stabilization.
Suitable additives generally have two typical structural features: firstly, such products contain one or more so-called “pigment-affinic” groups – anchor groups or adhesive groups – that all together provide a strong, durable adsorption onto the pigment surface. Secondly, such products contain binder-compatible chains which, after adsorption of the additive onto the pigment surface, protrude as far as possible from the pigment into the surrounding binder solution.
This layer of adsorbed additive molecules with the protruding polymer chains brings about the stabilization effect described above and therefore the deflocculation of the pigments. The effect is further intensified by the fact that the polymers of the coating binder interact with the polymer segments of the additive and can strengthen the adsorption layer.
When the pigment particles approach each other, the polymer segments penetrate and this causes steric stabilization.
Through specific structural elements composed of pigment-affinic groups (polar) and binder-compatible chains (less polar), these additives exhibit definitive surfactant properties. In other words, they not only stabilize the pigment dispersion by means of steric hindrance, but they also function as wetting additives. It is therefore not necessary to add a dose of special wetting additives when using these wetting and dispersing additives.
To ensure an effective stabilization, the additive envelope around the pigment particles should be as thick as possible – a thickness in excess of around 10 nm is generally considered sufficient. This means that the polymer segments of the additive must be as highly solvated as possible, i.e. they must have a good compatibility with the surrounding binder solution. If there is a poor compatibility, the polymer segments will fold together and then will not lie closely on the pigment surface: the stabilization against flocculation is then marginal. When selecting an additive for a specific system it is necessary to consider the compatibility between the additive and the binder.
The mechanism of steric stabilization can be applied both to aqueous as well as non-aqueous systems; of course, the additives must have the appropriate compatibility. While the electrostatic stabilization functions virtually only in aqueous systems, this restriction does not apply to steric stabilization.
On this website we use cookies and similar functions to process end device information and personal data. The processing is used for purposes such as to integrate content, external services and elements from third parties, statistical analysis/measurement, personalized advertising and the integration of social media. Depending on the function, data is passed on to up to 9 third parties and processed by them. This consent is voluntary, not required for the use of our website and can be revoked at any time using the icon on the bottom left.