
The dissociated ionic structures in the solid surface and selective ion adsorption cause dispersed solid particles in a liquid phase to carry electric charge. As the entire system is electrically neutral, the appropriate number of counter ions must be present in the adjacent liquid. We talk about an electrical double layer, which is made up of an adsorptive bound layer and a diffuse layer, in accordance with Stern’s model.
When two particles get close to one another, the double layers influence each other; in the case of opposite electrical charge they are attracted to each other, and with like charges they repel each other. The interaction between these electrostatic forces and the attracting London-van der Waal forces are described by the DLVO theory.
Additives can significantly influence the surface charge of the pigment particle: a targeted generation of strong charges brings about a high repulsion potential and thereby suppresses flocculation. Polyelectrolytes are particularly suitable as dispersing additives which function in this manner. Their polymer structure enables them to be readily and sustainably adsorbed on the pigment surface, and their multitude of ionic groups brings about considerable surface charges.
This type of stabilization is basically restricted to aqueous systems, as only here (on account of the high dielectric constant of the water) can sufficiently strong charges be produced. In principle, this mechanism also functions in organic solvents, however the surface charges are much lower, i.e. the thickness of the electrical double layer is considerably reduced and not usually sufficient to effectively prevent flocculation in the majority of cases.
Alongside the dielectric constant, the ion concentration and, above all, the valency of the ions have a strong influence on the electrical double layer. High ion concentrations and multivalent ions (even at a low concentration) can significantly worsen the stabilization and even cause it to completely fail.
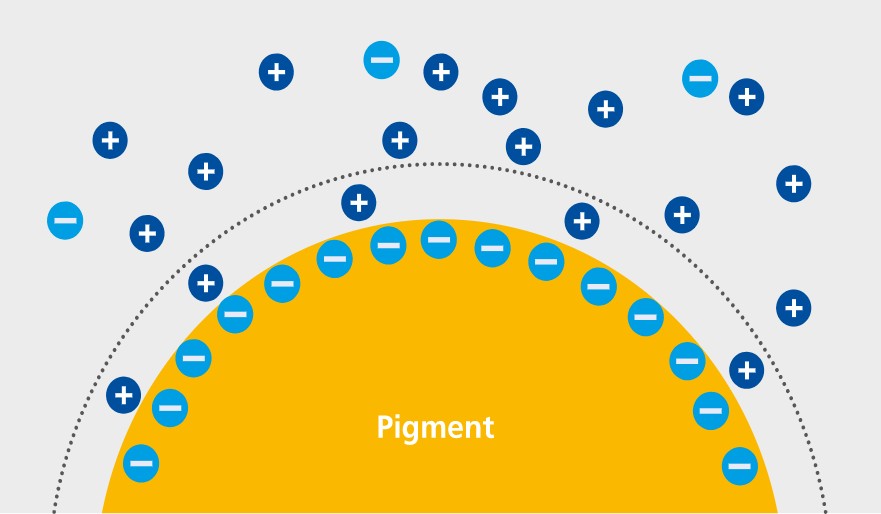
Adsorptive bound layer (Stern layer) and diffuse layer
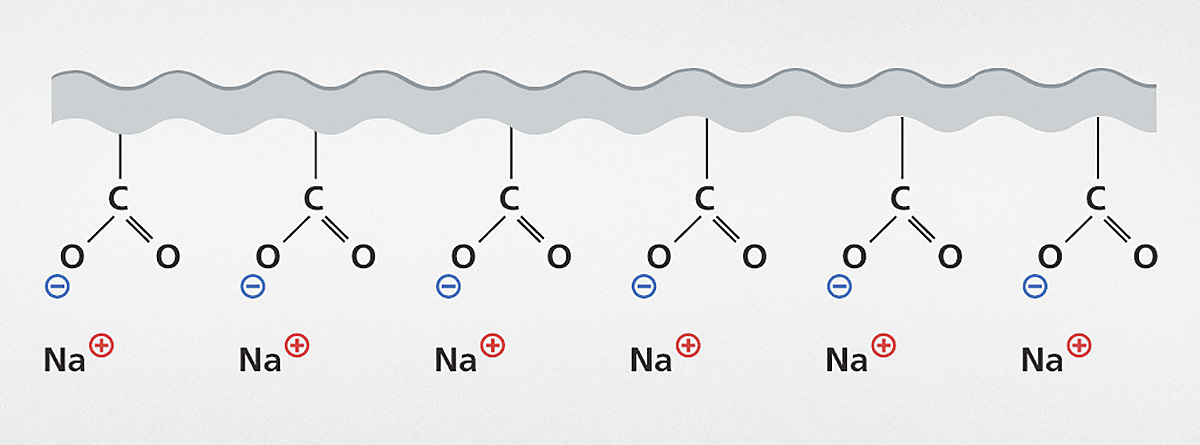
The standard dispersing additives used in the coatings industry which use electrostatic effects are polyphosphates and polyacrylates as a potassium, sodium or ammonium salt.
Alongside the influence on the charge, in some cases (dependent upon the polymer structure) a contribution towards stabilization by means of steric effects can be observed. The structure of the polyacrylates is similar to that of binders, therefore influencing the dried films less than the polyphosphates would. The phosphates have the advantage that they are also ideal for chelating multivalent ions (e.g. calcium) in the system and thereby eliminating the negative influence of these ions on the stabilization mechanism.
Dispersing additives of this type have been used successfully in aqueous emulsion paints for decades and continue to be used successfully today.
Polyelectrolyte-based dispersing additives for aqueous systems are pure dispersing additives and feature virtually no pigment wetting properties. Therefore, if the pigment wetting is also to be improved, they must be combined with the appropriate wetting additives.
On this website we use cookies and similar functions to process end device information and personal data. The processing is used for purposes such as to integrate content, external services and elements from third parties, statistical analysis/measurement, personalized advertising and the integration of social media. Depending on the function, data is passed on to up to 9 third parties and processed by them. This consent is voluntary, not required for the use of our website and can be revoked at any time using the icon on the bottom left.